Molnupiravir Price In India
The company has a US. 4hrs Chinese transgressions testing India.

Hetero Labs Seeks Emergency Nod For Covid 19 Drug Molnupiravir Hindustan Times
An oral antiviral drug appears to cut the risk of hospitalization and death from COVID-19 by roughly 50 percent in people newly diagnosed with the infection and at risk for severe disease pharmaceutical company Merck announced Friday morning.

Molnupiravir price in india. Said that its experimental COVID-19 pill reduced. 2 days agoMolnupiravir is designed to introduce errors into the genetic code of the virus and would be the first oral antiviral medication for Covid-19 news agency Reuters said. 1 day agoSecurity has been tightened in a northern Indian town after eight people including four protesting farmers died on Sunday.
The drug-maker and its partner Ridgeback Biotherapeutics released top-line results of a. Canon is finally unveiling full details of its top-of-the-line EOS R3 mirrorless camera today including specs price and availability. India is likely to allow the export of domestically produced doses of Russias Sputnik Light COVID-19 vaccine this month as the shot has yet to be approved in India two sources with knowledge of the matter told Reuters.
Violence broke out in Lakhmirpur Kheri district of Uttar Pradesh state during a protest against agricultural reforms. 2 days agoMerck recently announced that oral antiviral drug molnupiravir significantly reduced the risk of hospitalization or death at a planned interim analysis of the Phase 3 MOVe-OUT trial At the interim. Mercks Molnupiravir Zydus antibody cocktail and Glenmarks nasal spray are among more than 20 new drugs in different stages of drug trials or approvals in India to treat Covid-19.
Local media have reported that Indian drugmaker Hetero has already manufactured 2 million doses of the single-shot vaccine for whose approval the countrys drug. MSN Labs begins phase 3 trials for Molnupiravir 26 May 2021 0430AM IST MSN Labs begins Phase III trials of Molnupiravir on mild to moderate Covid-19 patients. 1 day agoBENGALURU Reuters - Indias Cipla has signed a deal with Eli Lilly to sell and distribute two of the US.
India adds 23529 Covid-19 cases 311 deaths in 24 hrs. All COVID-19 therapies now authorized in the US. Molnupiravir development codes MK-4482 and EIDD-2801 is an experimental antiviral drug which is orally active and was developed for the treatment of influenza.
An experimental COVID-19 treatment pill called molnupiravir being developed by Merck Co. Davis said Merck has similar agreements with other governments and is in. While the market for these drugs may not be huge at present due to a drop in Covid-19 cases and extended.
Government contract to supply 17 million courses of molnupiravir at a price of 700 per course. Davis said Merck has similar agreements with othergovernments and is in. The company has a US.
Novavax and Serum Institute of India file regulatory submission for World Health Organization Emergency Use Listing of Novavax recombinant nanoparticle protein-based COVID-19 vaccine GAITHERSBURG. An anonymous reader quotes a report from Ars Technica. The R3 will be available in India simultaneously with global.
The California biotech furthermore shared with investors that the study drug absolutely destroyed molnupiravir demonstrating 10-fold 10x higher antiviral potency targeting not only wild type SARS-CoV-2 but also variants of interest including Alpha UK and Delta India while superior to the Merck drug showing 3 to 7x higher antiviral. The company will focus its pandemic efforts on advancing molnupiravir and on producing Johnson Johnsons COVID-19 vaccine. The company has a US government contract to supply 17 million courses of molnupiravir at a price of US700 per course.
We shared that the drug manufacturer Merck made the decision to discontinue development of MK-7110 for COVID-19. One promising drug that could improve things is molnupiravir an antiviral thats moving into the final stages of testing in humans. India Slaps Reciprocal Travel Curbs on COVID-19 Vaccinated UK Nationals - Source NEW DELHI Reuters - UK nationals visiting India this month will.
Novavax and Serum Institute of India file regulatory submission for World Health Organization Emergency Use Listing of Novavax recombinant nanoparticle protein-based COVID-19 vaccine GAITHERSBURG. Government contract to supply 17 million courses of molnupiravir at a price of 700 per course. Adds details of earlier trial and Phase III treatment study adds share price By Deena Beasley.
Molnupiravir an experimental antiviral pill developed by Merck Co could halve the chances of dying or being hospitalized for those most at risk of contracting severe COVID-19 according to data that experts hailed as a potential breakthrough in how the virus is treated. Price of diesel breaches 100 in State. The drug was developed at Emory University by the.
Sept 29 Reuters - Laboratory studies show that Merck Cos experimental oral COVID-19 antiviral drug molnupiravir is likely to be effective against known variants of the coronavirus including the dominant highly transmissible Delta the company said on Wednesday. If cleared Mercks drug - molnupiravir - would be the first pill shown to treat COVID-19 a potentially major advance in efforts to fight the pandemic. Drugmakers best-selling diabetes treatments in the country the companies said in a.
Davis said Merck has. TrialSite reported on this drugs unique journey to development. Molnupiravir development codes MK-4482 and EIDD-2801 is an experimental antiviral drug which is orally active and was developed for the treatment of influenzaIt is a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine and exerts its antiviral action through introduction of copying errors during viral RNA replication.
Require an IV or injection. It is a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine and exerts its antiviral action through introduction of copying errors during viral RNA replication. A drug like molnupiravir the name is a reference to Thors hammer Mjölnir could also help compensate for persistent gaps in Covid-19 vaccination coverage both in.
Molnupiravir The Miraculous Drug To Treat Covid 19
Natco Pharma Seeks Approval For Covid Drug Candidate Molnupiravir Shares Jump 7
Us Charges Glenmark Pharma With Conspiracy In Price Fixing
Molnupiravir Seeking A Pill To Cure Covid Drugmakers Eye Alternative To Vaccines Health News Et Healthworld

Covid 19 Medicine Price Reduced To 75 Per Tablet By Glenmark Pharma

Favicovid Favipiravir 200 Mg Tablets 10 10 Treatment Covid 19 Id 23361837073

Antiviral Drug Molnupiravir Blocks Covid 19 Virus Within 24 Hours Study Youtube
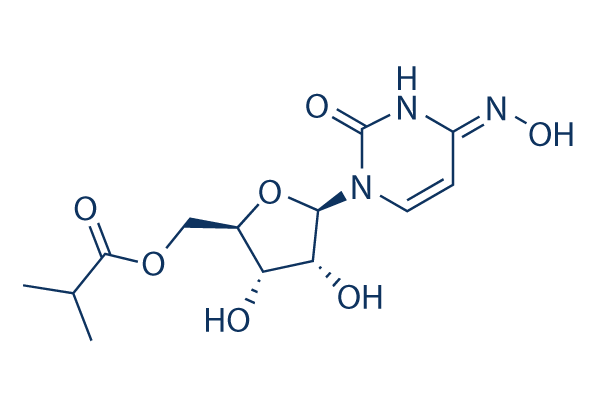
Molnupiravir Eidd 2801 99 Hplc Selleck Sars Cov Inhibitor

Natco Seeks Cdsco Approval For Phase Iii Trial Of Molnupiravir For Covid 19 Treatment The Financial Express
News Announcements Natco Pharma

From Merck S Molnupiravir To Glenmark S Nasal Spray Over 20 New Drugs In Pipeline For Covid 19

Molnupiravir 200mg Tab For Oral Packaging Size 40 Tablets In Hdpe Bottle Id 23702721573

200 Mg Molnupiravir Prescription Treatment Covid 19 Rs 10 Box Id 23374050730

An Oral Pill For Covid 19 Molnupiravir Shows Promise Stella
Molnupiravir Emerges As Latest Gamechanger Drug For Covid 19 India News India Tv
Optimus Pharma Gets Dcgi Nod To Conduct Phase 3 Trial Of Covid 19 Antiviral Drug Molnupiravir
Coronavirus Five Firms Come Together For Trial Of Covid 19 Drug Molnupiravir The Hindu



Komentar
Posting Komentar